Morningstar's 2014 CEO of the Year
Gilead's John Martin has established leading market share and spectacular profitability for the innovative biotech firm.
We are pleased to announce that John Martin is the 2014 Morningstar CEO of the Year. Under his leadership, Gilead Sciences' focus on infectious disease has paid off tremendously. With a small salesforce, inexpensive manufacturing, and selective research and development, the company generates stellar profit margins and is extending its reach from HIV into other high-margin markets such as hepatitis C and hematological oncology.
Martin, who is also Gilead’s chairman, has shown exemplary stewardship in the company's moat-building investment strategies, good allocation of capital, and superior board independence and qualifications. Gilead's moat was formed by its leadership position in the treatment of HIV, with three patented products that are today's most popular regimens. Despite numerous competitors, the company has established leading market share and spectacular profitability with its convenient, effective, and safe treatments. Gilead now serves about 85% of treated HIV patients in the United States. We think hepatitis C drug Sovaldi and the recently approved combination regimen Harvoni are both redefining Gilead as a powerhouse in the broader infectious-disease market. Sovaldi led the way for all-oral treatments in the multibillion-dollar hepatitis C market, where we expect that Gilead has longevity extending as far as 2029.
Martin is the only insider on Gilead's 11-member board, which has an independent lead director. Martin, who was previously Bristol-Myers Squibb’s director of antiviral chemistry and has more than a quarter-century of experience, replaced Gilead's founder as CEO in 1996. We like that management is rewarded for research and development progress rather than earnings per share. Gilead's decision to boost share repurchases has been a smart one, in our view, as shares have traded below our fair value estimate over the past two years.
Smart Acquisitions and New Treatment Areas Ensure Strong Returns Key patents begin to expire in 2018, but we think the firm has shown that it can translate its extensive understanding of the drug discovery and development process in HIV into new therapeutic areas. Patent protection on newer HIV regimens as well as the recent U.S. Food and Drug Administration approval of Sovaldi (sofosbuvir) should be enough to ensure strong returns for the next couple of decades, making visibility on profits clearer at Gilead than at many of its large-cap biotech peers. We expect Gilead's hepatitis C sales to come in close to $12 billion for 2014, or roughly 70% of our global market estimate for the year. Gilead's expertise in infectious diseases and single-pill formulations is a part of its R&D strategy, which we see as one of the strongest intangible assets supporting the firm's wide moat.
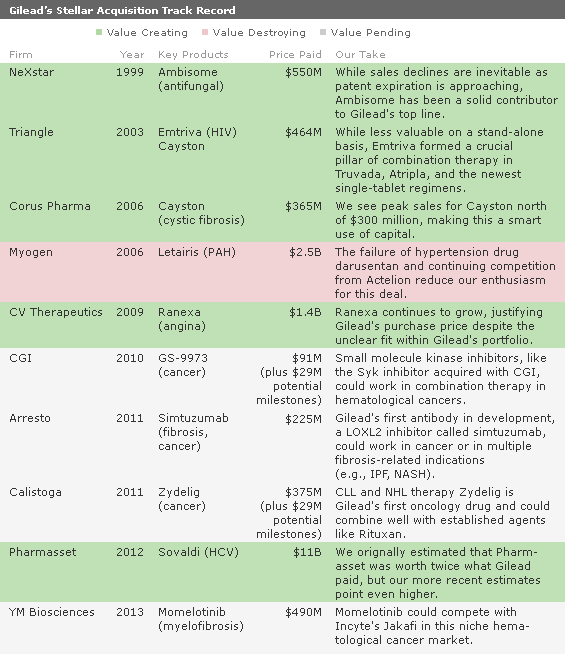
Martin and his team have completed acquisitions and collaborative deals over the years that have bolstered the company's infectious-disease portfolio. The acquisition of Triangle in 2003 brought Emtriva, a critical component of Truvada and all of the firm's single-tablet HIV regimens. Though outside of Gilead's therapeutic area focus, the acquisition of CV Therapeutics was also a wise investment, as angina drug Ranexa (ranolazine) is growing strongly. Gilead's first cancer drug, Zydelig (idelalisib), launched in 2014, and we forecast almost $3 billion in peak sales in chronic lymphocytic leukemia and non-Hodgkin lymphoma combined.
Leading the Way in Hepatitis C Treatment With Game Changer Sovaldi Our investment thesis rests on the (now largely proven) theory that the $11 billion bet on Pharmasset in 2012--and Sovaldi--was an excellent use of capital. Though this initially looked like a pricey deal for a development-stage asset, it garnered the most valuable hepatitis C drug in the industry and demonstrated the firm's recognition of Sovaldi's potentially unique safety and efficacy profile compared with other, toxic nucleotide analogs. We think the firm's experience with another nucleotide analog, tenofovir, a key ingredient in all of Gilead's HIV combination regimens, probably contributed to its recognition of Sovaldi's value at an early stage in its development.
We think the low resistance potential and pan-genotypic efficacy of Sovaldi will allow Gilead to retain a significant portion of its dominant market share after the late 2013 launch, despite emerging competition from firms like AbbVie and Bristol. Sovaldi saw $8.5 billion in sales in the first nine months of 2014, and the Sovaldi/ledipasvir combination Harvoni was launched in the United States in October. Harvoni, for 12 or potentially even eight weeks of therapy, looks like the leading option for patients with genotype 1, the most prevalent genotype in the United States and Europe. Twelve weeks of Sovaldi with standard hepatitis C drug ribavirin is more effective than interferon/ribavirin in genotypes 2 and 3, and Gilead has a large pipeline of drug candidates that could allow it to serve a range of genotypes without the use of ribavirin, and with an even shorter duration of all-oral therapy.
Legacy HIV Franchise Continues to Shine Management has done an excellent job of maximizing sales of the tenofovir molecule, which is present in Viread, Truvada, and all single-tablet regimens, forming the heart of the firm's $9 billion-plus HIV franchise. Its newest single-tablet regimens, Complera and Stribild, are seeing rapid uptake and strong reimbursement. Such regimens offer patients convenience and affordability, as patients are less likely to miss doses and develop drug resistance, and they only need to make one copayment. Gilead will see new competitive threats in HIV; GlaxoSmithKline recently received FDA approval for a single-tablet regimen called Triumeq and could introduce a single-tablet regimen built on Truvada once its patents begin to expire in 2018. In addition, generic versions of Atripla should be available after 2021. However, we think Complera and Stribild will have a strong grasp on the market by this time, resetting the firm's HIV patent cliff into the 2020s. Gilead has an updated tenofovir (TAF) in its pipeline that appears to have bone and renal safety advantages over the older tenofovir, and Gilead has filed for FDA approval of its first single-tablet regimen based on TAF. We also see potential for other TAF-based regimens, which could lengthen Gilead's HIV profits.

/author-service-images-prod-us-east-1.publishing.aws.arc.pub/morningstar/558ccc7b-2d37-4a8c-babf-feca8e10da32.jpg)
/d10o6nnig0wrdw.cloudfront.net/10-04-2024/t_e6175f671cee439d9180e460f6081183_name_file_960x540_1600_v4_.jpg)
/cloudfront-us-east-1.images.arcpublishing.com/morningstar/LE5DFBLC5VACTMC7JWTRIYVU5M.jpg)
/cloudfront-us-east-1.images.arcpublishing.com/morningstar/PJQ2TFVCOFACVODYK7FJ2Q3J2U.png)
:quality(80)/author-service-images-prod-us-east-1.publishing.aws.arc.pub/morningstar/558ccc7b-2d37-4a8c-babf-feca8e10da32.jpg)