FDA Clears Clarius OB AI for Handheld Ultrasound: A Leap Forward in Prenatal Care
FDA Clears Clarius OB AI for Handheld Ultrasound: A Leap Forward in Prenatal Care
PR Newswire
VANCOUVER, BC, June 25, 2024
The company's 8th AI model makes ultrasound easier for new ultrasound users to capture important fetal measurements
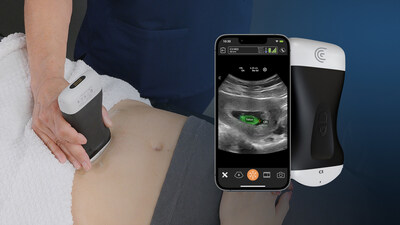
VANCOUVER, BC, June 25, 2024 /PRNewswire/ -- Clarius Mobile Health, a leading provider of high-definition handheld ultrasound systems, has obtained FDA clearance for the Clarius OB AI fetal biometric measurement tool, improving access to obstetrical (OB) prenatal monitoring and care in resource-limited areas. The OB AI model automatically performs fetal biometry measurements to estimate fetal age, weight and growth intervals and is available now with the Clarius C3 HD3 wireless handheld ultrasound scanner in the United States and Canada.
Developed with state-of-the-art deep learning models leveraging more than 30,000 de-identified fetal ultrasound images, Clarius OB AI provides consistent and precise measurements enabling new ultrasound users, including midwives and nurses, the ability to perform accurate fetal ultrasound measurements with confidence.
"When I used Clarius OB AI while teaching OB ultrasound to a group of midwives, I was impressed by the accuracy of cursor placement on multiple scans, from crown rump length to biparietal diameter and femur length," says Carolyn L. Gegor, an ARDMS certified midwife sonographer. "I found that after using the OB AI model, the students were more able to properly measure structures without the assistance of the AI. It was a quality improvement and time saver for me as a skilled scanner and an excellent teaching modality for those without previous scanning experience."
Two-thirds of women worldwide lack access to prenatal ultrasound for monitoring wellbeing1. While ultrasound imaging is widely recognized as the gold standard for capturing accurate biometric measurements to monitor fetal wellbeing, the high cost of equipment, lack of portability, and the extensive training required are barriers to widespread adoption by midwives and nurses. By integrating AI with affordable and specialized ultrasound technology, Clarius aims to broaden ultrasound access for midwives and nurses, to help improve maternal and fetal health outcomes.
"OB AI is a testament to Clarius' commitment to innovation in the handheld ultrasound market," said Clarius President and CEO Ohad Arazi. "Our mission is to make high-quality ultrasound technology accessible and user-friendly, and with OB AI we're pleased to be helping more clinicians provide accurate and efficient prenatal assessments, ultimately improving maternal and fetal health outcomes."
OB AI is included with Clarius membership. Once activated on the Clarius app while a clinician is scanning the abdomen, OB AI automatically highlights key fetal anatomy and places calipers to calculate measurements, which appear on screen and can be added to a patient's electronic record from the app. Clarius OB AI is the 8th AI model developed in-house that is available with Clarius ultrasound scanners.
On July 23, Clarius is hosting a practical webinar designed specifically for midwives with midwifery expert and ultrasound educator Carolyn Gegor. The one-hour webinar will focus on the role of first trimester point–of–care ultrasound in early pregnancy care. Registration is open now for the free webinar.
About Clarius Mobile Health
Clarius is on a mission to make accurate, easy-to-use, and affordable ultrasound tools available to all medical professionals in every specialty. With decades of experience in medical imaging, the team knows that great ultrasound imaging improves confidence and patient care. Today, AI-powered Clarius handheld wireless ultrasound scanners connect to iOS and Android devices, delivering high-resolution ultrasound images traditionally only available with bulkier, high-end systems at a fraction of the cost. Almost 4 million high-definition scans have been performed using Clarius wireless handheld scanners. Clarius scanners are available in over 90 countries worldwide. Learn more at www.clarius.com.
Clarius Media Contact
Marie-Claire Charlton
Director of Marketing
379592@email4pr.com
Phone: +1 (778) 800-99754 ext. 148
1 Mariani G, Kasznia-Brown J, Paez D, Mikhail MN, H Salama D, Bhatla N, Erba PA, Kashyap R. Improving women's health in low-income and middle-income countries. Part II: the needs of diagnostic imaging. Nucl Med Commun. 2017 Dec;38(12):1024-1028. doi: 10.1097/MNM.0000000000000752. PMID: 28953209; PMCID: PMC5704652.

SOURCE Clarius Mobile Health
-
What’s Happening In the Markets This Week
-
Obesity Drug Stocks: Why It Will Be ‘Exceptionally Difficult’ to Dethrone Eli Lilly and Novo Nordisk
-
What Does Chipotle’s Stock Split Mean for Investors?
-
5 Stocks to Buy Before the Fed Cuts Interest Rates in 2024
-
Markets Brief: Inflation Is Back In the Spotlight
-
What a Strong Economy Now Means for the Rest of 2024
-
4 Wide-Moat Stocks to Buy for the Long Term While They’re Undervalued Today
-
Markets Brief: Four Stocks Made Up 80% of the Gains. Can It Last?
-
The 10 Best Companies to Invest in Now
-
Nike Earnings: Dim Sales Outlook Slams Shares, but Patient Investors Could Be Rewarded
-
2 Top E-Commerce Stock Picks
-
Our Top Pick for Investing in US Renewable Energy
-
Micron Earnings: We Raise Our DRAM Forecast and Valuation Behind Stronger Pricing Assumptions
-
10 Undervalued Wide-Moat Stocks
-
What Is a Stock Split?
-
10 Best Value Stocks to Buy for the Long Term